- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
X-ray Crystallography
Protein structure generation and analysis
To generate an X-ray crystal structure, samples are first crystallised and then an X-ray beam is used to create a diffraction pattern from which the position of each atom in the crystallised molecule is determined. Domainex has extensive expertise and the facilities in-house to undertake high-throughput screening of thousands of crystallisation conditions. Through a network of world-class synchrotron facilities across Europe, with whom we have established access agreements, including the Diamond Light Source (Oxfordshire, UK), we will obtain high-resolution datasets for your target proteins (both apo or ligand-bound).
X-ray crystallography is currently the gold-standard in terms of protein/protein-ligand structure generation and can be applied to proteins with a broad range of molecular weights. Data collection and structure refinement is more straightforward than for the complementary technique of cryogenic electron microscopy (Cryo-EM) and the resolution is typically higher.
For examples that showcase our capabilities in X-ray crystallography please see our MEK-1, CD73 and G9a case studies.
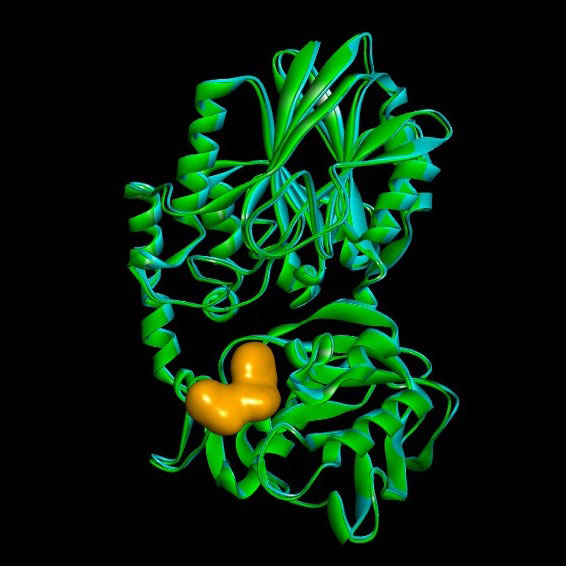
Figure 1: X-ray crystal structure of CD73 bound to adenosine 5′-(α,β-methylene)diphosphate (AMPCP) a CD73 inhibitor (dataset resolution 1.7Å). The structure overlays well with the Apo open conformation structure which was also solved.
For proteins which are not amenable to X-ray crystallography (for instance large complexes, membrane proteins or proteins which do not crystalise readily), we also offer Cryo-EM services.


Image kindly provided by the Diamond Light Source
Case studies
Start your next project with Domainex
Contact one of our experts today