- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Thermodynamic Solubility Assay
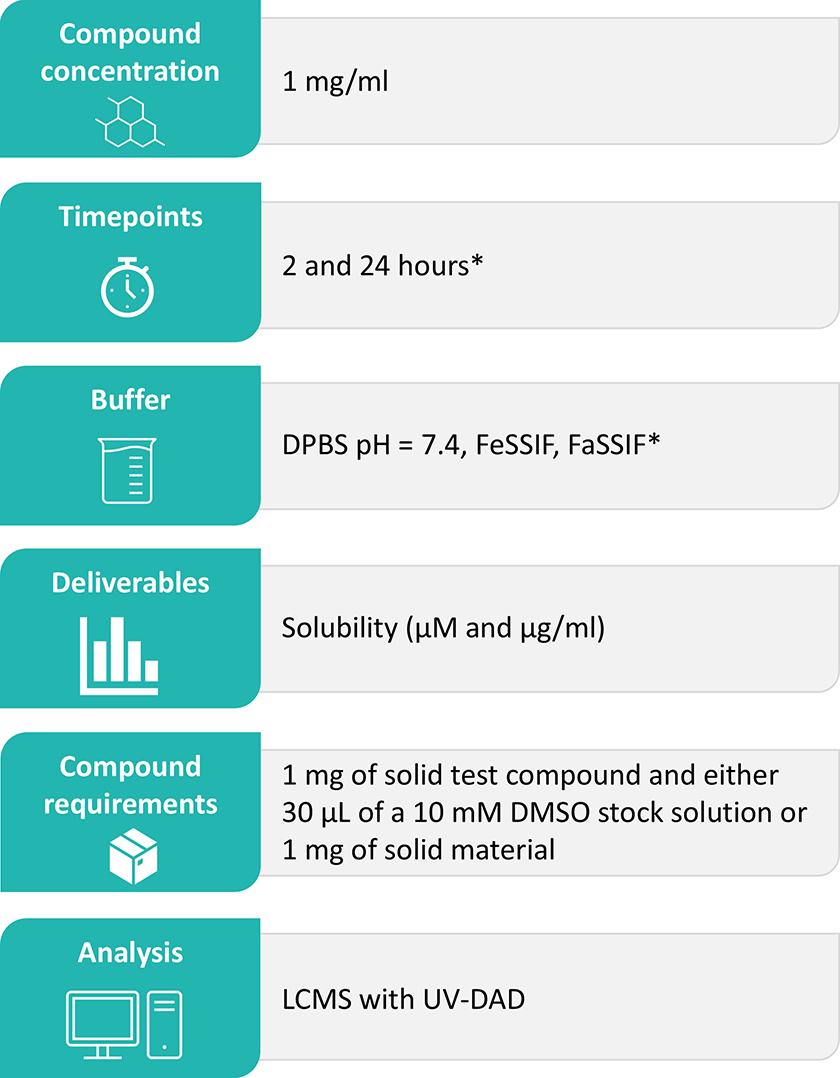
In vitro assays require test compounds to be in aqueous solution, so it is important for them to be sufficiently soluble at assay concentrations. Domainex offers thermodynamic solubility in Dulbecco's phosphate-buffered saline (DPBS) at pH 7.4, Fed State Simulated Intestinal Fluid (FeSSIF), and Fasted State Simulated Intestinal Fluid (FaSSIF) as standard. Other buffers may be used upon request. FeSSIF and FaSSIF solubility is indicative of solubility in the gastrointestinal tract, which will affect bioavailability following oral uptake.
Domainex’s Standard Experimental Procedure:
Diluent is added to the solid test sample and the solution is shaken for the relevant length of time before centrifugation. The supernatant is filtered and analysed by Liquid Chromatography Mass Spectrometry (LCMS) with UltraViolet (UV)-Diode Array Detection (DAD) using a calibration curve.
Data Analysis
UV absorbance is detected using LCMS with a UV-DAD instrument. The UV-DAD affords both sensitivity and versatility, allowing monitoring over a wide range of the UV-visible spectrum. The following equation is used to calculate amount of sample (µg) on column:

The solubility value is obtained by dividing sample (µg) on column by injection volume.
*Other options available upon request

Figure 1: Plots showing UV absorbance of aqueous solution (left) and DMSO standard (right) at different injection volumes.
Deliverables
The results are reported in Excel file format as solubility (µM and µg/ml) calculated for each timepoint assessed. Any relevant comments about compound stability/solubility/etc. are also included in the report.
Turnaround time from receipt of the compounds to release of data is typically 2 weeks.
Start your next project with Domainex
Contact one of our experts today