- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
GSH Reactivity Assay
The glutathione (GSH) reactivity assay can be used to assess structure-reactivity relationships of cysteine-targeting covalent inhibitors. The half-life of adduct formation with GSH can be a valuable tool in fine-tuning the reactivity of these inhibitors. The GSH reactivity assay can be performed using single test compounds or as pools of 5* to accommodate higher throughput screening.
*Conditional on the mass difference being >5 Da between compounds in the same pool
Domainex’s Standard Experimental Procedure:
Test compounds are incubated with GSH in the presence of ethylenediaminetetraacetic acid (EDTA) at 37 °C over a period of 8 hours. The reactivity of compound with GSH is assessed in real time using Ultra-High Performance Liquid Chromatography (UHPLC) connected to a mass spectrometer. Both the depletion of test compound and appearance of the expected GSH adduct are monitored. The stability of test compound in PBS after 8 hours is also assessed. Example data is shown in Figure 1 and Table 1.
Data Analysis:
The depletion of parent compound and the formation of GSH adduct are monitored using UHPLC-QToF or UHPLC-QDa/SQD instruments. The ultraviolet (UV) absorbance is used to determine the area under the curve, whilst the mass spectrum is used to confirm the nature of the species being monitored. Log linear plots of parent depletion allow the half-life of the test compounds to be calculated.

Assessment of test compound stability in PBS allows resolution between drug candidate stability and reactivity to be made.
*Other options available upon request
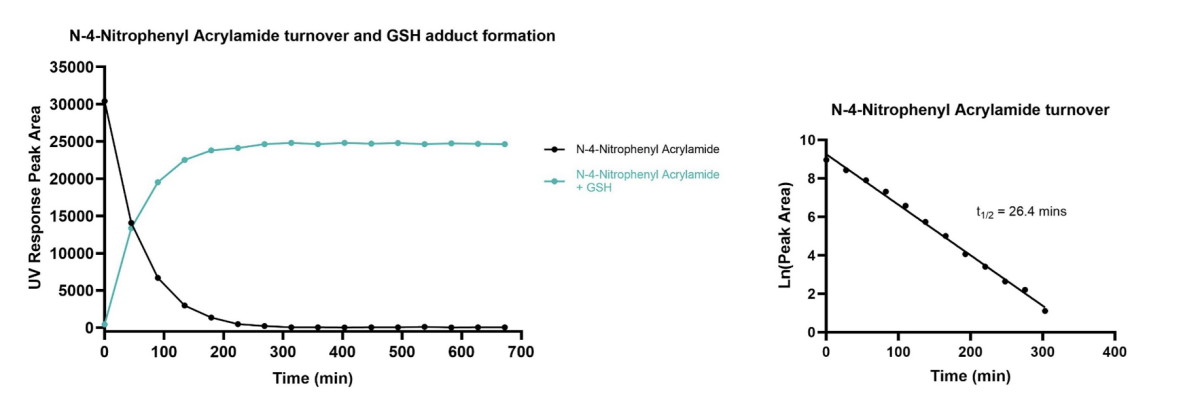
Figure 1: Left: Plot showing depletion of positive control standard and GSH adduct formation. Right: Natural log plot allows determination of compound half-life.
Deliverables:
The results are reported in Excel file format as t1/2 (min) and percentage recovery in PBS (%) values. Confirmation of whether glutathione adduct formation has been detected is provided. Any relevant comments about compound stability/solubility/etc. are also included in the report.
Turnaround time from receipt of the test compounds to release of data is typically less than 2 weeks.
Reversible Covalent Inhibitors
Most covalent inhibitors are irreversible, forming permanent covalent bonds with their target proteins. While this often leads to desired effects such as long-lasting activity, it can also give rise to significant issues later in the drug discovery pipeline, e.g. off-target toxicity. In contrast, reversible covalent inhibitors overcome the challenge of off-target toxicity by forming temporary covalent bonds, allowing for the their release from off-target proteins, thereby limiting their potentially toxic effects, while still achieving effective treatment duration.
In addition to assessing irreversible inhibitors, our GSH reactivity assay has been adapted and optimised to identify the presence of a reversible covalent inhibitors by establishing an equilibrium between the GSH adduct and the parent compound. By incorporating an additional step, a reversible reaction can be confirmed by monitoring for adduct depletion and parent re-formation upon GSH release.
With the popularity of covalent inhibitors increasing, this enhanced GSH assay provides an invaluable tool to not only assess a compound’s reactivity but also the reversibility of the reaction.
For more information on Domainex’s reversible GSH assay, please visit see our Reversible Covalent Inhibitors poster.

Figure 1. Exemplar real-time kinetics data for an irreversible covalent inhibitor showing depletion of parent compound and GSH adduct formation.

Figure 2. Exemplar real-time kinetics data for a reversible covalent inhibitor showing depletion of parent compound and GSH adduct formation.
Start your next project with Domainex
Contact one of our experts today