- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- DMPK
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Plasma Stability Assay
Plasma stability is an important part of the ADME screening cascade. Instability of test compounds in plasma can lead to rapid in vivo clearance and poor pharmacokinetics. Plasma stability assessments alert medicinal chemists to any such structural liabilities and therefore provide the opportunity to remove or modify these structural features before the series progresses further. Certain types of chemical classes (e.g., esters, amides, sulphonamides and lactones) are particularly susceptible to transformation catalysed by enzymes present in plasma, and compounds containing these groups should be assessed at an early stage. Moreover, for prodrugs designed to be cleaved in plasma, determination of plasma stability can give valuable kinetic information, guiding the design of prodrugs with favourable properties.
Experimental Procedure:
Test compounds and assay standards are incubated in plasma at 37°C for two hours. Aliquots of plasma are removed at the chosen timepoints, and the proteins are precipitated by the addition of an organic solvent. Supernatants are diluted further, and depletion of test compound is monitored using UPLC-MS/MS. Example data is shown in Figures 1 and 2.
Data Analysis:
Extracts are analysed using Waters Acquity UPLC TQ-S, TQ-S micro or TQ-XS instruments. Triple quadrupole mass spectrometers operated in multiple reaction monitoring (MRM) mode, provide accurate measurements with excellent sensitivity, selectivity and reproducibility.
Plotting the natural logarithm (ln) of compound response against time allows the determination of half-life (t1/2) using following equation:

Deliverables:
The results are reported in Excel file format as half-life (min), including standard error of mean (SEM), and compound remaining (%) at the end of the incubation. Any relevant comments about compound stability, solubility or binding are also included in the report as comments.
Typical turnaround time from receipt of test compounds to release of data is ten working days or less.
*Other options available upon request
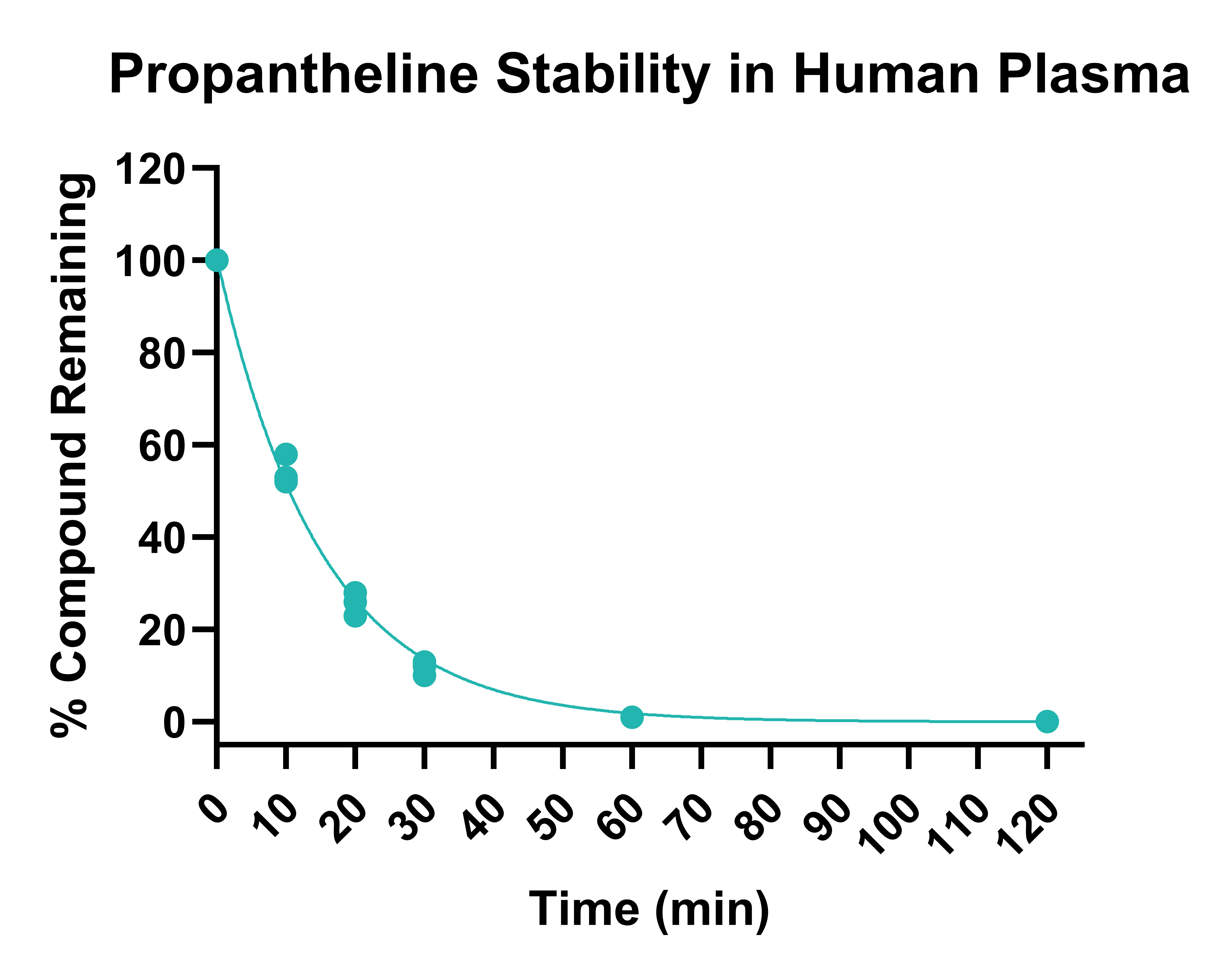
Figure 1. Stability of propantheline in human plasma following 120 min incubation, determined on three different occasions.
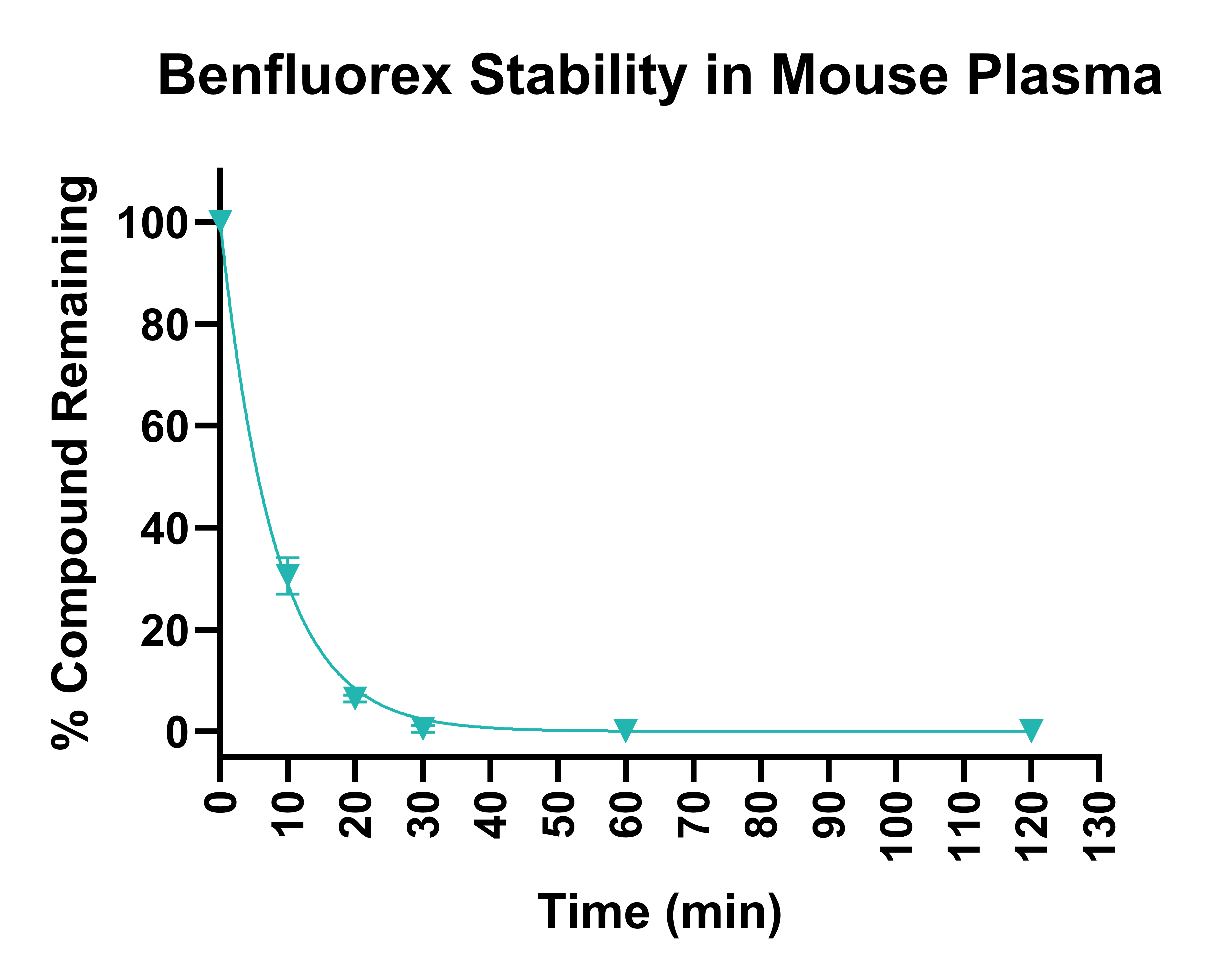
Figure 2. Stability of benfluorex in human plasma following 120 min incubation, determined on three different occasions.
Start your next project with Domainex
Contact one of our experts today