- About
-
Solutions
-
Services
- Biosciences
- Chemistry
- Integrated Drug Discovery
- Computer Aided Drug Design
- Hit Identification
- Target Classes and Modalities
- Therapeutic Areas
-
A-Z
- A
- B
- C
- D
- E
- F
- G
- H
- I
- K
- L
- M
- N
- O
- P
- R
- S
- T
- V
- X
-
Services
- Library
- News & Events
- Careers
Shake Flask LogD
Lipophilicity is an important parameter that can have a direct effect on intestinal absorption, distribution into tissues, metabolism and clearance of a candidate drug. Highly lipophilic compounds can lead to low bioavailability and poor exposure. LogD, the distribution co-efficient, is a measure of lipophilicity. The partition of a compound between an organic solvent and an aqueous phase in both ionised and neutral forms can be measured at any pH. Domainex’s LogD7.4 assay determines the compounds propensity to partition into lipid-rich environments by mixing the sample in n-octanol and phosphate buffered saline (PBS) at pH 7.4, followed by shaking for a fixed length of time and quantification by Liquid Chromatography-Mass Spectrometry (LC-MS) with an UltraViolet-Diode Array Detector (UV-DAD).
Domainex’s Standard Experimental Procedure:
Test compounds are prepared in n-octanol pre-saturated with PBS (pH 7.4) and PBS (pH 7.4) pre-saturated with n-octanol. The mixture is vigorously shaken for a fixed period of time to ensure equilibrium between the two phases has been reached. The mixture is then centrifuged to achieve phase separation. The upper organic and lower aqueous layers are filtered before analysis by Liquid LCMS with UV-DAD. A range of injection volumes are used to generate response curves. This assay is most suitable for moderately acidic to moderately basic compounds (considering the partition is a function of the pH).
Example data is shown in Figure 1.
* Other options available upon request

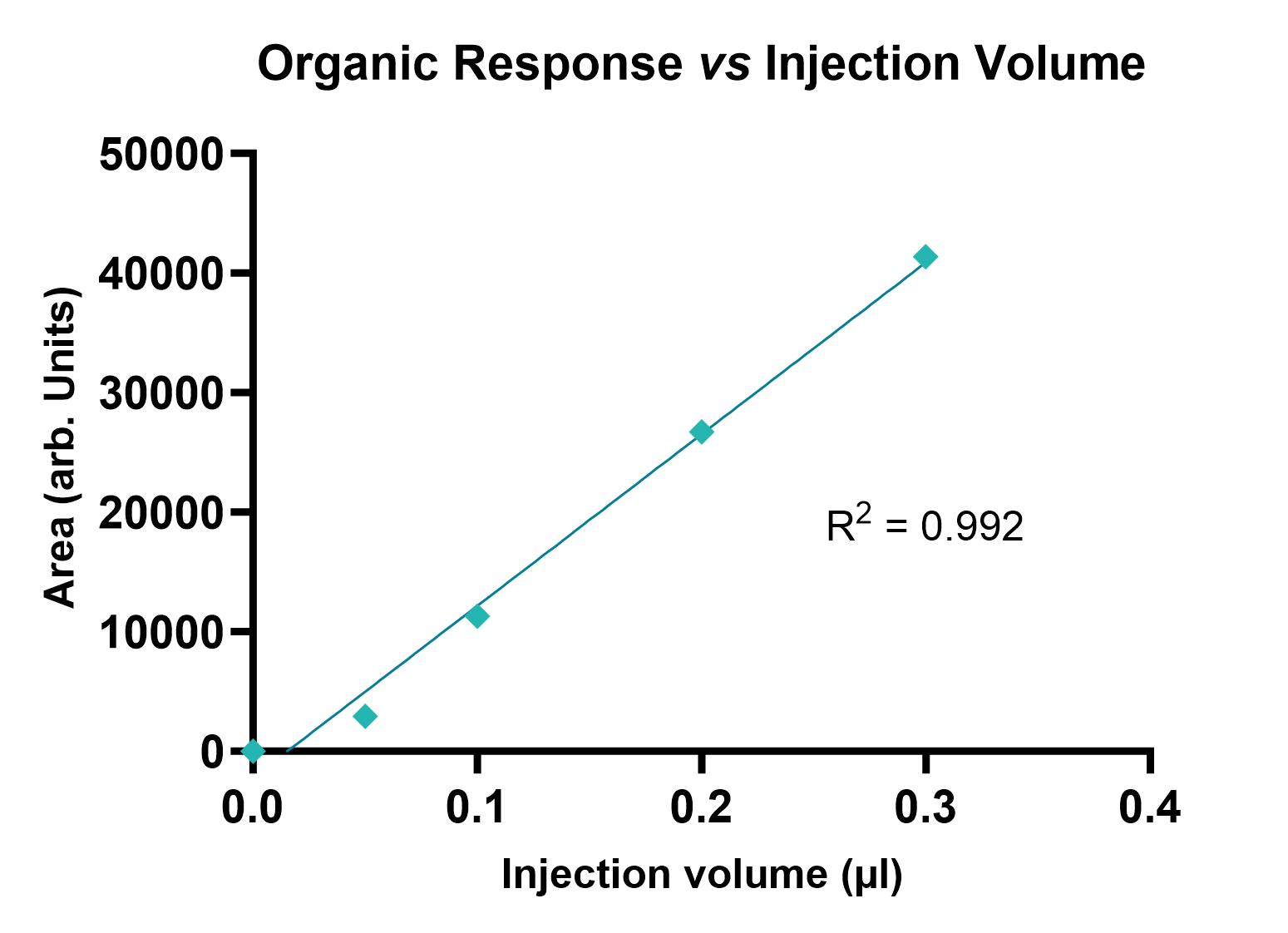
Figure 1: Plots showing UV absorbance of PBS pH 7.4 (left) and n-octanol (right) fractions at different injection volumes.
Data Analysis:
UV absorbance is detected using LCMS with a UV-DAD instrument. The UV-DAD affords both sensitivity and versatility, allowing monitoring over a wide range of UV-visible spectrum. The following equation is used to calculate LogD7.4:

The Log D7.4 value is obtained as a ratio of compound in the n-octanol and PBS phases.
Deliverables:
The results are reported in Excel file format as calculated LogD values. Any relevant comments about compound stability/solubility are also included in the report.
Turnaround time from receipt of the test compounds to release of data is typically 2 weeks.
Start your next project with Domainex
Contact one of our experts today