By Gabriel Negoita-Giras (Team Leader, Chemistry)
The VHL (von Hippel-Lindau) protein is well known for being an important regulator within the hypoxic stress response mechanism and its inhibition could be beneficial in treating anaemia. It is also implicated in the recruitment of the Cullin RING E3 ligase, thus being directly involved in the ubiquitination and protein degradation machinery. As a consequence, VHL is one of the most commonly targeted proteins by bivalent degraders within the area of Targeted Protein degradation (TPD).
VH032 and VH021 are two of the most widely utilized VHL ligands (Figure 1), but due to their peptidic nature, they suffer from poor cellular permeability. This in turn translates to low oral bioavailability, thus limiting their applicability in vivo. With this in mind, scientists at Genentech set out with the aim of improving the potency, and equally importantly, the oral bioavailability of VHL ligands.1
Their strategy focussed on the systematic modification of the LHS and RHS portions of the ligands, while leaving the hydroxy-proline (Hyp) core unmodified to retain recognition by VHL protein (Figure 1). Target engagement was measured in a cellular NanoBRET® (Promega) assay in the absence and presence of the cell permeabilising agent digitonin, to obtain permeability data and VHL binding potency data in parallel, thus enabling both parameters to be optimised simultaneously.
To improve synthetic tractability a terminal RHS amide was introduced, the RHS portion was elongated by one C atom, while the thiazole group was replaced by a phenyl ring to boost permeability. This yielded compound 5 (Figure 1) as a suitable starting point for optimisation of the LHS (the thiazole RHS would be reintroduced and the terminal amide removed at a later stage of the optimisation process).
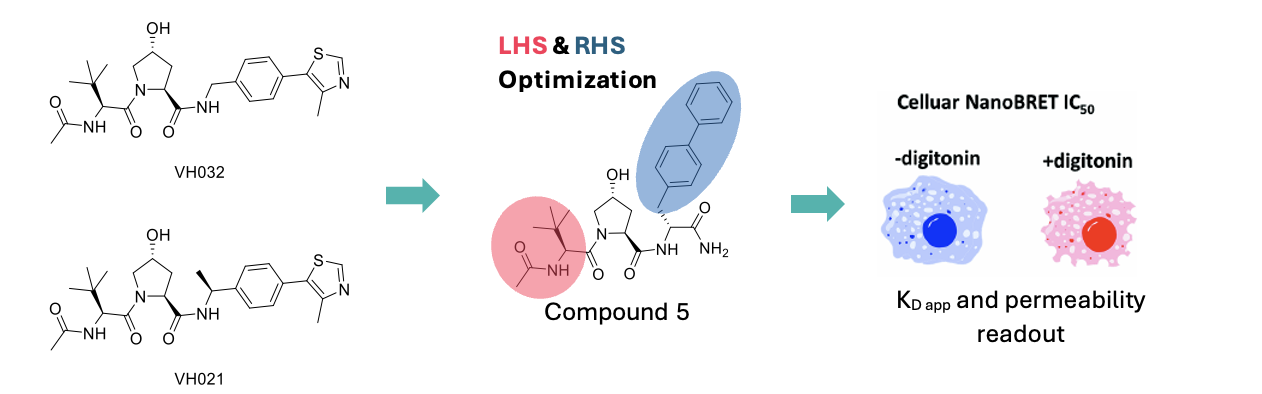
Figure 1. VHL commercial ligands (VH032 and VH021) and the optimisation strategy employed by Wu et al. The BRET signal difference observed between experiments performed in presence/absence of digitonin can be correlated with compound permeability.1
In terms of the LHS, the first approach was to examine the effect of replacing the tBu sidechain of tert-butylglycine. The SAR indicated that only the (S)-enantiomer was tolerated, while bulky, lipophilic substituents correlated well with increased potency. Conformational analysis helped rationalise the observed SAR by indicating that larger substituents were able to restrict the torsion angles of the side chain effectively favouring a bioactive conformation. This culminated in the identification of the adamantly group as optimal, and yielded the most potent analogue in the series (compound 44, Figure 2).
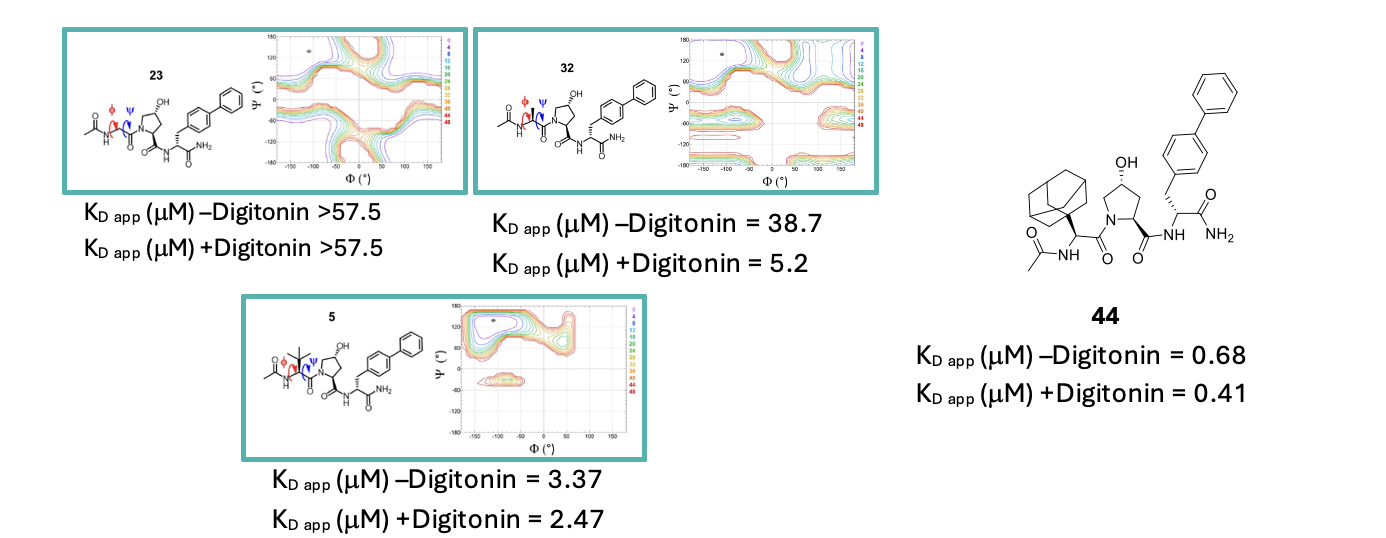
Figure 2. Torsion angle analysis of glycine substituent effect on conformation (bulky groups limit rotation and restrict conformation to the bioactive one) and structure of compound 44.
Another strategy employed in the optimisation of the LHS was to replace the terminal acetamide with a variety of substituted and unsubstituted 5 and 6-membered ring bioisosteres. As a result of this exercise, compound 90, a cyclo-propyl substituted triazole analogue (Figure 3) was identified as the optimal LHS modification.
Combining the optimised LHS modification with the VH032 thiazole RHS resulted in hybrid analogue 104 with improved potency. Activity gains were rationalised by solving the crystal structure of compound 104 and VHL which highlighted several novel interactions between the triazole LHS and the protein (Figure 4) not observed with VH032.
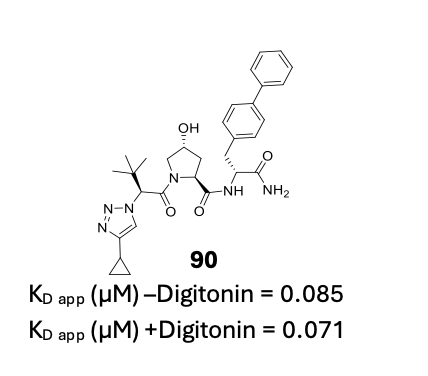
Figure 3. Structure of compound 90
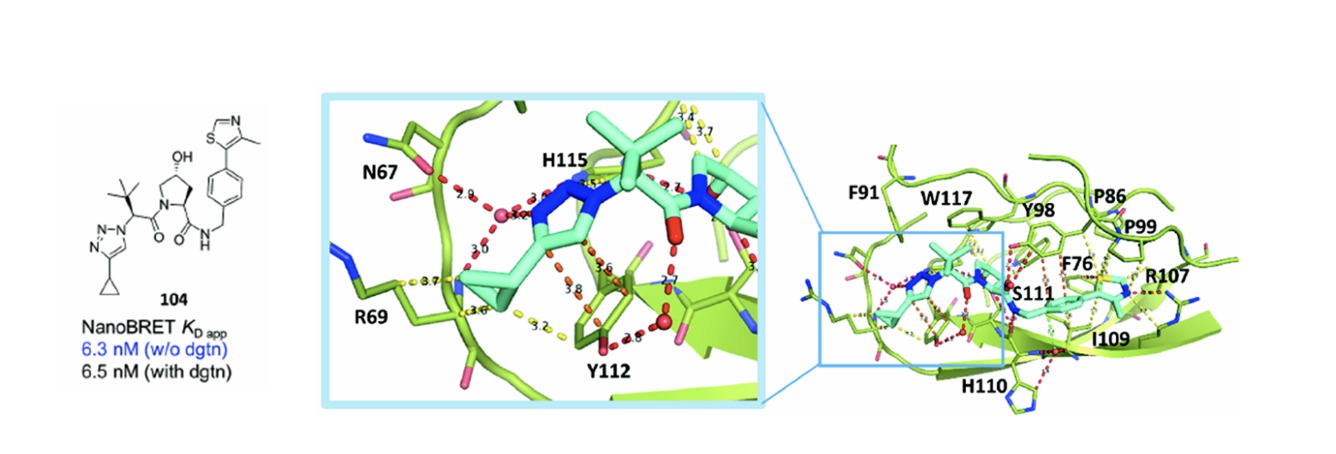
Figure 4. Structure of compound 104 and the interactions of the optimised LHS with VHL
Further improvements in potency were achieved by conformationally restricting the RHS of the molecule. A seven membered tethered system proved optimal, as it was discovered to lock the molecule into the bioactive conformation, yielding compound 107, also known as GNE7599 (Figure 5).
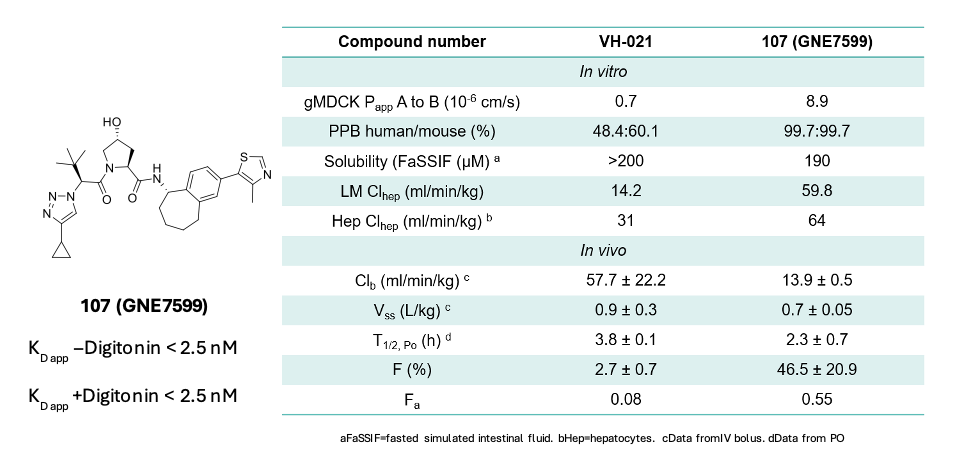
Figure 5. Structure of GNE7599 and its PK properties compared to that of VH021
Next, the in vitro and in vivo PK profiles of GNE7599 were determined and compared to its closest commercial analogue (VH021). It was found that GNE7599 has a 10-fold improved permeability in a MDCK cellular assay compared to VH021 (Figure 5). It is highly protein bound in plasma and has a moderate metabolic stability in both microsomes and hepatocytes. In vivo, while the Vss is similar, the clearance is reduced significantly which can be rationalised by the high PPB, which imparts a protective effect. These combined properties led to an excellent oral bioavailability of 47% (vs 2.7% for VH021, a 17-fold improvement).
In summary, by employing a systematic peptidomimetic SAR strategy, the scientists at Genentech successfully identified a series of optimised VHL binding ligands. Their efforts culminated in the discovery of GNE7599, which exhibits >120-fold improved potency vs VHL-021 and much improved cellular permeability which has translated to good oral bioavailability in in vivo PK experiments. No doubt GNE7599 will serve as a valuable tool compound in future VHL-mediated degrader programmes.
At Domainex, we are passionate about TPD and are experts in designing, synthesising and profiling heterobifunctional degrader molecules. If you would like to get in touch to discuss your TPD project, please contact us here.
Reference:
- Wu H, Murray J, Ishisoko N, Frommlet A, Deshmukh G, DiPasquale A, Mulvihill MM, Zhang D, Quinn JG, Blake RA, Fairbrother WJ, Fuhrmann J. Potency-Enhanced Peptidomimetic VHL Ligands with Improved Oral Bioavailability. J Med Chem. 2024 Jun 13;67(11):8585-8608.