By Chris Matheson (Group Leader, Chemistry)
Our attention was recently drawn to two manuscripts published by the Early Oncology group at AstraZeneca in the Journal of Medicinal Chemistry disclosing the discovery of two series of prodrugs of arginase inhibitors.1,2 Arginase is an attractive target in immuno-oncology due to its role in depleting arginine concentrations in the tumour microenvironment (TME), a level of which is required for T cell proliferation and, hence, the innate immune response. Two isoforms of arginase exist - Arg1 and Arg2 – both of which share high sequence homology and comparable function but differ in tissue distribution. Arg1 and Arg2 catalyse the hydrolysis of arginine to ornithine and urea, which occurs in a particularly hydrophilic active site. This site contains a high concentration of Asp, Glu and His residues and two manganese atoms (Figure 1), which are required to bind and stabilize arginine and the hydrolysis transition state, both of which are highly polar.
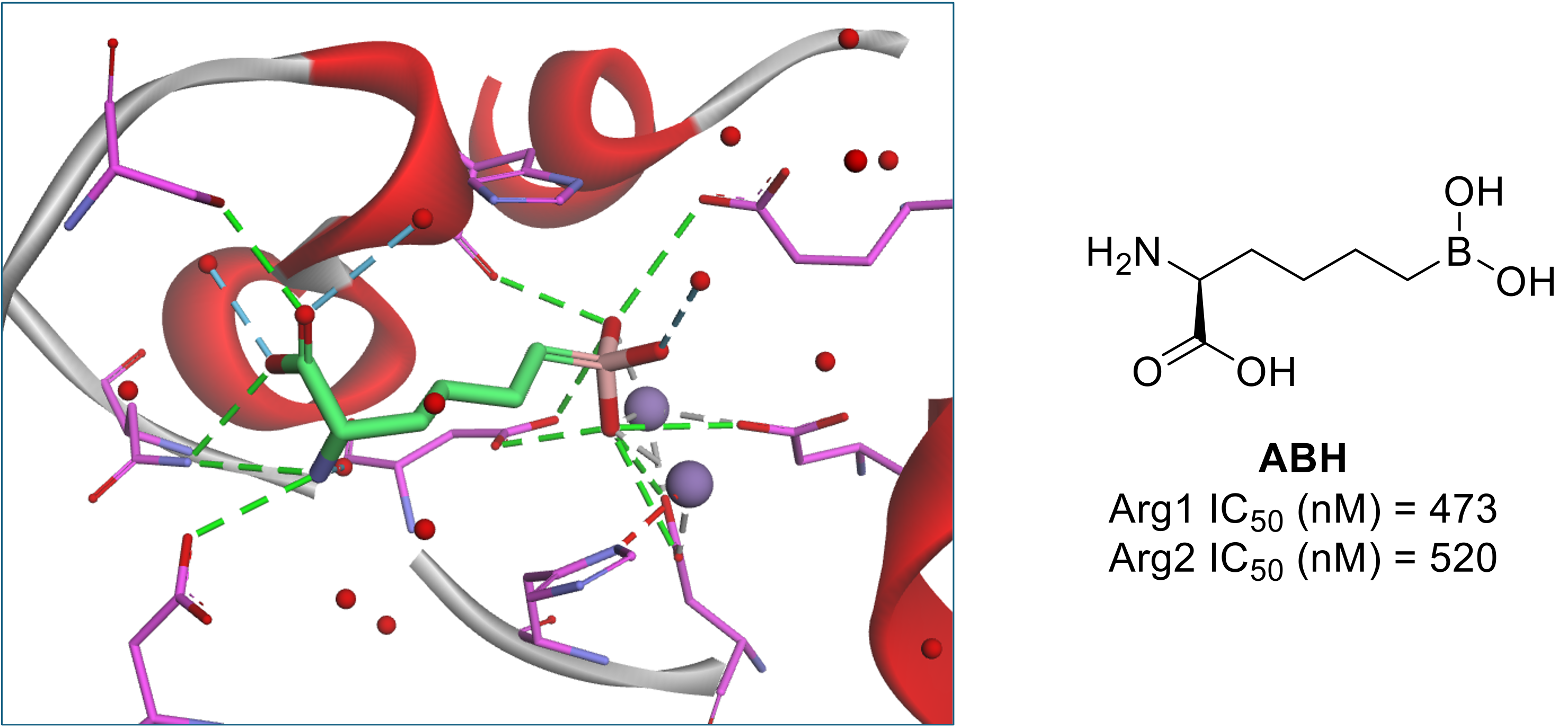
Figure 1: Active site of Arg2 (residues magenta, Mn atoms purple) with boronic acid-based inhibitor ABH (green) bound (PDB: 1D3V).
Due to the nature of the active site, existing ligands for arginase have been characterised by high polarity, existing outside of classical rule-of-five physicochemical property space. One such inhibitor, 2(S)-amino-6-boronohexanoic acid (ABH, Figure 1), acts as the starting point for many arginase inhibitors, including those described in these papers. The boronic acid moiety of ABH is able to form a tetrahedral borate species via incorporation of the active site catalytic hydroxide unit and has been found to be critical to the potency of this compound class, with efforts to change this functionality proving unsuccessful.
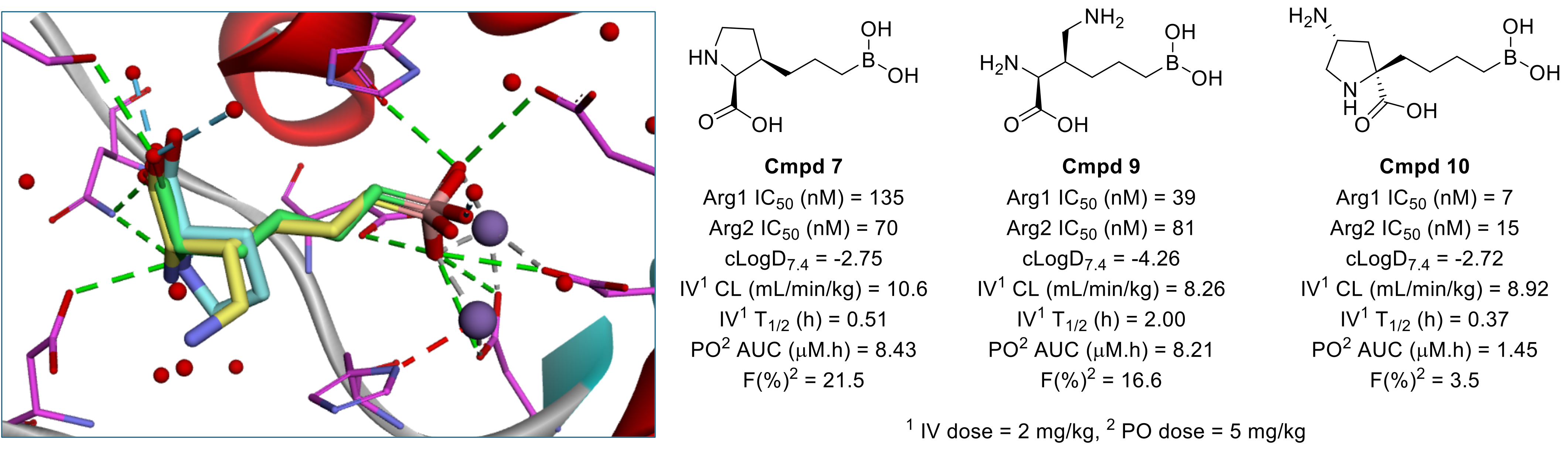
Figure 2: Overlay of crystal structures of ABH (green, PDB:1D3V), proline (cyan, PDB: 8RGU) and compound 9 (yellow, PDB: 8RFA) bound to Arg2.
In their efforts to improve the potency of boronic acid based arginase inhibitors, the team at AstraZeneca noted that the amino acid portion of ABH overlayed well with proline bound to Arg2 (Figure 2, PDB: 8RGU), leading to the hybrid compound 7, which exhibited improved potency against both Arg isoforms. Switching from a β-substituted proline to an α-substituted proline was initially detrimental to activity (data not shown), but observation that the amino group of their most potent acyclic arginase inhibitor compound 92 overlayed with the pyrrolidine C4 ultimately led the team to the aminoproline analogue 10 (Figure 2).
Despite excellent potency, compound 10 suffered from a common trait of arginase inhibitors in this series – poor oral bioavailability, due to the hydrophilic nature of the binding site requiring high polarity (compound 10: F(%) = 3.5, cLogD7.4 = -2.72). Importantly, arginase is secreted into the TME and so efficacious compounds require oral bioavailability but are not required to permeate the tumour itself. Noting the similarity between compound 10 and natural dipeptides, the team sought to exploit PepT1/2 transport mechanisms to increase the systemic exposure of the compound and used the known pharmacophore model of PepT1/2 substrates to identify the pyrrolidine-NH2 as the optimal attachment point for a series of cleavable amino acids (to generate prodrugs). Comparing IV and PO in vivo PK data, the researchers were able to correlate steric bulk of the amino acid with IV CL, used as a surrogate for hydrolytic stability and payload release (Table 1), although it was noted that there appeared to be some enzymatic process at play due to the relative stability of glycine analogue 20.
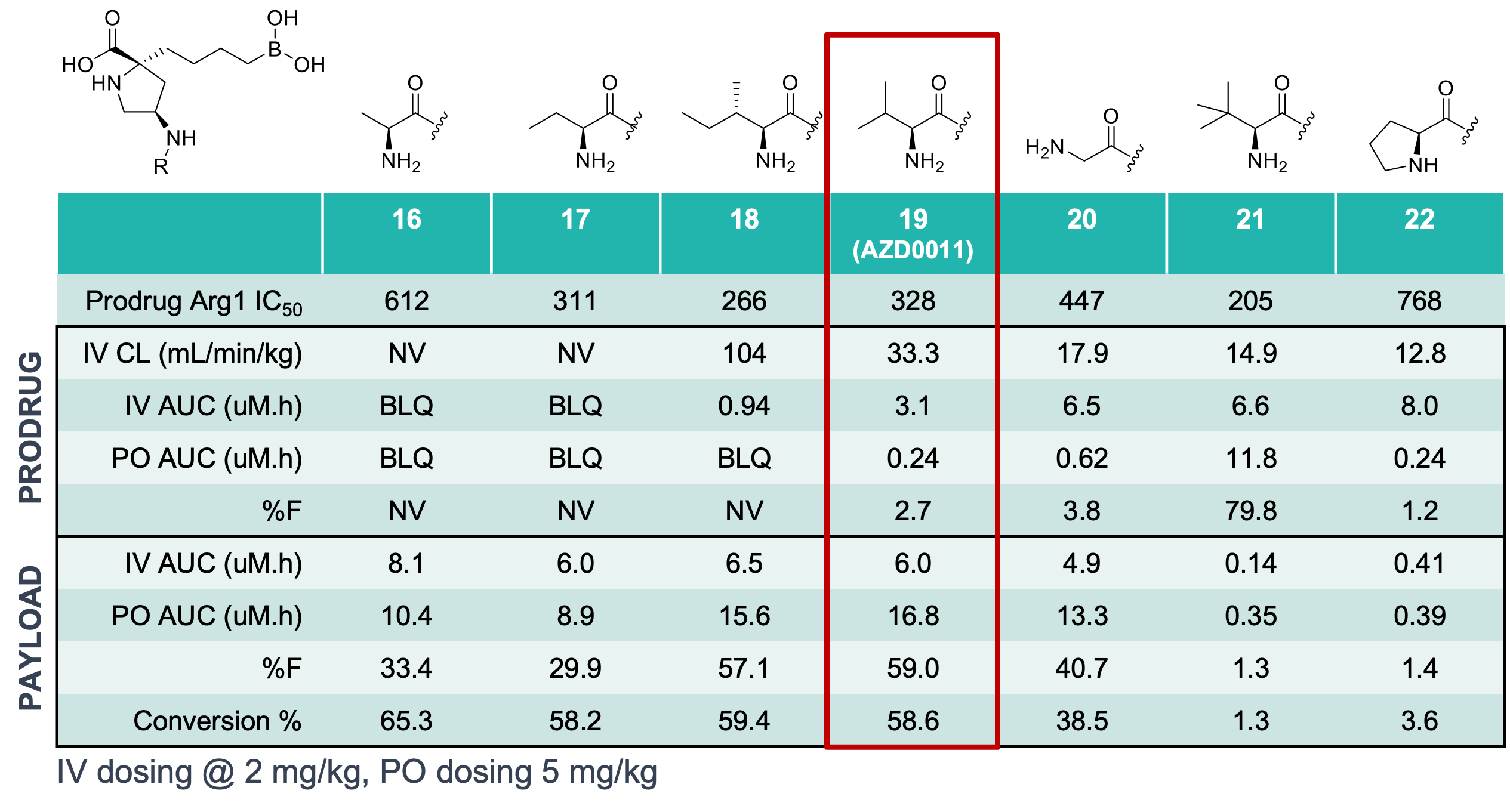
Table 1: In vivo PK data following a single dose of prodrugs 16-22, payload rows monitoring for compound 10. BLQ = below limit of quantitation, NV = no value.
The sweet spot balancing pre-systemic stability, active transport and release of the payload was obtained with compound 19, now termed AZD0011, which was further assessed for in vivo efficacy. A dose dependent increase of compound 10 exposure was observed, with no saturation of PepT1/2 transport mechanisms, and was mirrored with an increase in arginine concentrations and concomitant decrease in ornithine concentrations both in plasma and the TME, consistent with arginase inhibition. Variable results were observed for AZD0011 in a Ag104Ld xenograft model, both as a monotherapy and in combination with an anti-PD-L1 antibody, with some animals lacking a therapeutic response while other animals showed minimal tumour growth or regression, highlighting the need for careful patient selection as can be common in immuno-oncology.
Achieving such promising in vivo data against a target usually plagued by inhibitors with poor oral bioavailability brings arginase into focus as a druggable target for immuno-oncology indications where elevated arginine levels are observed. We eagerly await further data on this series of prodrugs from the AstraZeneca team.
At Domainex we have extensive experience in the development of enzyme inhibitors (including kinases, methytransferases and proteases) and in the design and synthesis of prodrugs. Please get in touch if you would like to find out more.
References:
Discovery of (2R,4R)-4-((S)-2-Amino-3-methylbutanamido)-2-(4-boronobutyl)pyrrolidine-2-carboxylic Acid (AZD0011), an Actively Transported Prodrug of a Potent Arginase Inhibitor to Treat Cancer. Scott N. Mlynarski, Brian M. Aquila, Susan Cantin, Steve Cook, Aatman Doshi, M. Raymond V. Finlay, Eric T. Gangl, Tyler Grebe, Chungang Gu, Sameer P. Kawatkar, Jens Petersen, Petar Pop-Damkov, Alwin G. Schuller, Wenlin Shao, Jason D. Shields, Iain Simpson, Siavash Tavakoli, Sharon Tentarelli, Scott Throner, Haixia Wang, Jianyan Wang, Dedong Wu and Qing Ye. Journal of Medicinal Chemistry 2024 67 (23), 20827-20841.
Design and Synthesis of Acyclic Boronic Acid Arginase Inhibitors. Jason D. Shields, Brian M. Aquila, David Emmons, M. Raymond V. Finlay, Eric T. Gangl, Chungang Gu, Scott N. Mlynarski, Jens Petersen, Petar Pop-Damkov, Li Sha, Iain Simpson, Siavash Tavakoli, Sharon Tentarelli, Haixia Wang, Qing Ye and XiaoLan Zheng. Journal of Medicinal Chemistry 2024 67 (23), 20799-20826.