By the Domainex Synthesis Group (Hugh Tawell, Tenin Traore, Andrew Jones, Andrea Bombana, Venu Komanduri, Fergus Preston, Brahmam Medapi, Robyn Presland, and Vinny Duong)
In this edition of our blog series, we have focused on the following two recent publications, which describe highly useful synthetic transformations:
- Aminative Suzuki–Miyaura coupling. Polpum Onnuch, Kranthikumar Ramagonolla, and Richard Y. Liu, Science, 2024, 1019-1024, 383.
Copper-Catalysed Dehydrogenation or Lactonization of C(sp3)–H Bonds. Shupeng Zhou, Zi-Jun Zhang and Jin-Quan Yu, Nature, 2024, 363–369, 629.
Aminative Suzuki-Miyaura Coupling
The Suzuki-Miyaura coupling (SMC) and Buchwald-Hartwig coupling (BHC) reactions are widely implemented to install respectively C-C and C-N bonds. The reliability and generality of these cross-coupling reactions have considerably influenced how chemical space is probed in drug discovery, and therefore the structures of recently approved small-molecules. In their recent publication, Liu and co-workers reported the introduction of a formal nitrene insertion into the Pd-catalysed SMC reaction, modifying the product from C-C-linked biaryls to C-N-C-linked diaryl amines (Scheme 1).1 The applications of this methodology could potentially expand the product space of cross-coupling reactions and enhance structural diversity during the drug discovery process.
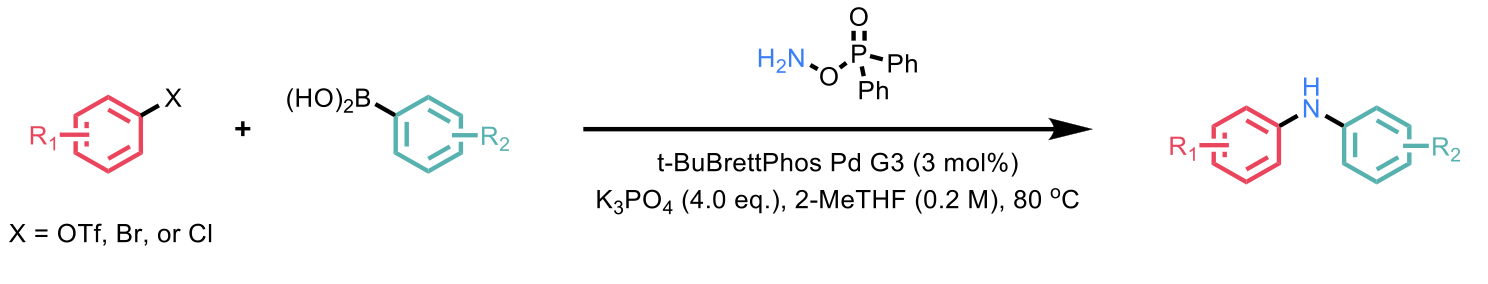
Scheme 1: Combination of SMC starting materials with an amination reagent to afford BHC-like products.
Initial efforts were focused on the coupling between a triflate and a boronic acid in presence of a range of electrophilic amination reagents as a precursor of the formal nitrene (“NH”) partner. The use of catalysts supported by phosphine ligands (i.e., RuPhos) showed complete conversion to the C-C-linked SMC product. When BrettPhos was used, a catalyst typically implemented in BHC reactions, the desired product was obtained in 70% yield and the SMC product in 36% yield. However, when a t-BuBrettPhos-modified Pd catalyst was used the desired product was obtained in 96% yield with only 3% of the SMC product.
The generality of this protocol is showcased by its effectiveness across a diverse range of (hetero)aryl triflates, as well as (hetero)aryl bromides and chlorides. Moreover, the relatively mild conditions make it compatible with various functional groups (e.g. nitro, nitrile, ortho-Me, ortho-alkoxy, ortho-halides, amide, α-acidic aryl ketones, alkyl ketones, and esters).
The authors demonstrated the applicability of this methodology by using it to synthesise NH-inserted variations of drug-like molecules such as Sonidegib and Etoricoxib, respectively a smoothened (SMO) inhibitor for the treatment of basal cell carcinoma and a cyclooxygenase-2 (COX-2) inhibitor for the treatment of arthritis pain. The authors did not comment on the selectivity between the BHC and the SMC products for these two reactions.
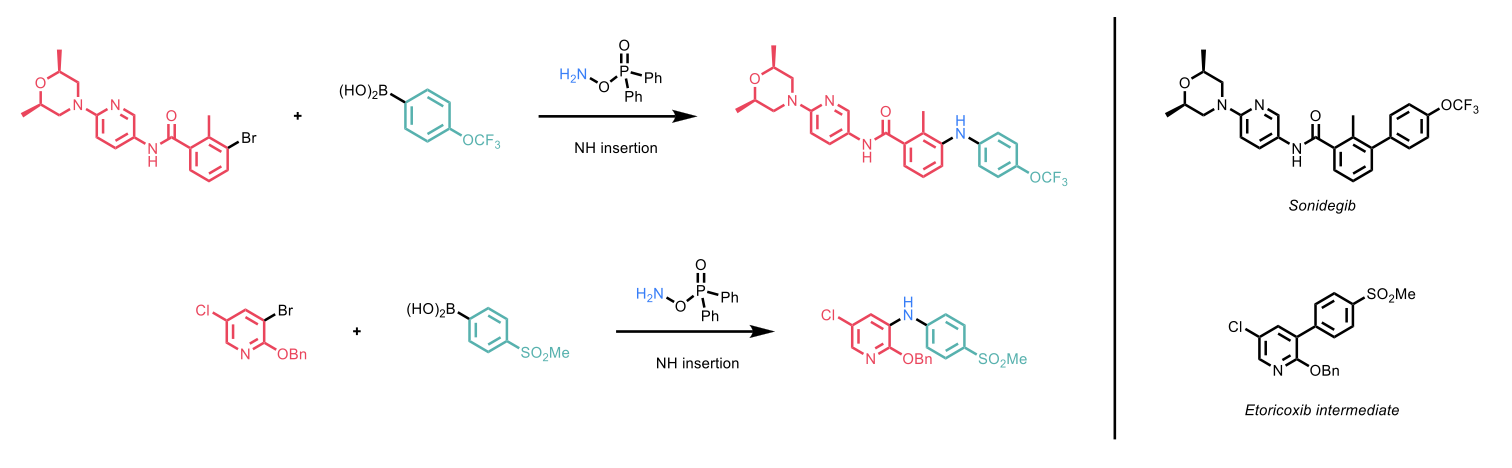
Scheme 2: The synthesis of analogues of Sonidegib and Etoricoxib with NH insertions.
In conclusion, the authors successfully developed a method to join the Suzuki-Miyaura and the Buchwald-Hartwig coupling pathways to achieve C-N-C-linked diaryl amines. In the future, the mechanistic flexibility of this reaction could be exploited to implement various classes of both nucleophiles and electrophiles.
Copper Catalysed Dehydrogenation or Lactonization of C(sp3)-H Bonds
Olefins and lactones are important functionalities in organic synthesis and accessing these directly via dehydrogenation/oxidation of C(sp3)-H bonds is a very elegant strategy. Inspired by the cytochrome P450 enzymes which are known to catalyse bimodal oxidation of aliphatic acids via radical intermediates; the authors demonstrated a highly regioselective dehydrogenation/oxidation of N-methoxyamide substrates using copper catalysis without an external oxidant.
The beauty and advantage of this method resides within the fact that it allows controllable, bimodal transformation as shown in Scheme 3 below.
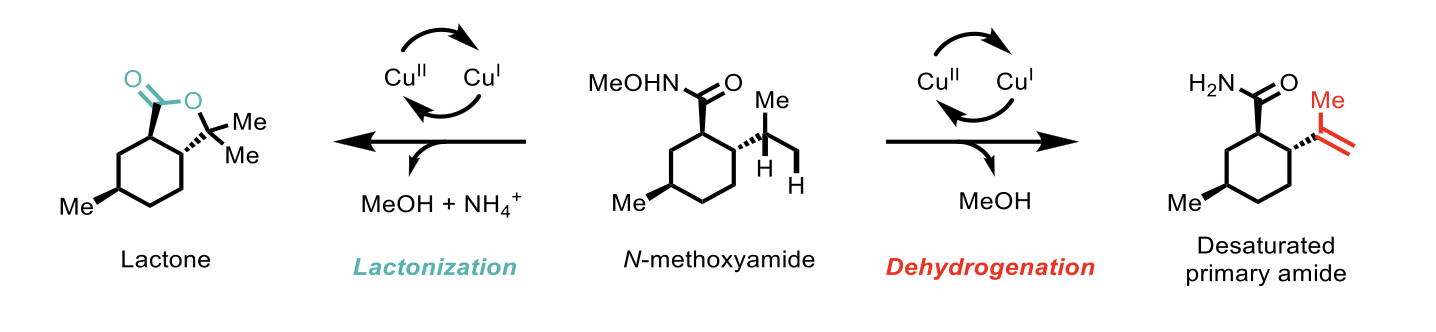
Scheme 3: Bimodal copper-catalysed oxidation for the rapid diversification of N-methoxyamides.
N-methoxyamide substrates were used to generate an amidyl radical in the presence of Cu(I) catalysts to give either a desaturated primary amide or a lactone, as shown in Scheme 3 above. Initial attempts for the dehydrogenation reaction using CuF2 catalyst (10 mol%), 8-methoxyquinoline ligand (20 mol%) and AcOH as an additive successfully gave the desired desaturated primary amide. Interestingly, traces of lactone were observed in some cases, and by using neat AcOH (or 1,4-dioxane) in the presence of TFA (5 eq.), the lactonization reaction became the more favoured one. During the study, the authors observed that the [Cu(MeCN)4]BF4 complex was superior to CuF2 catalyst for the lactonization. The reason for this is not clear but according to the authors a strong acid (i.e. TFA) in polar solvent favours the lactonization reaction by facilitating formation of the carbocation intermediate.
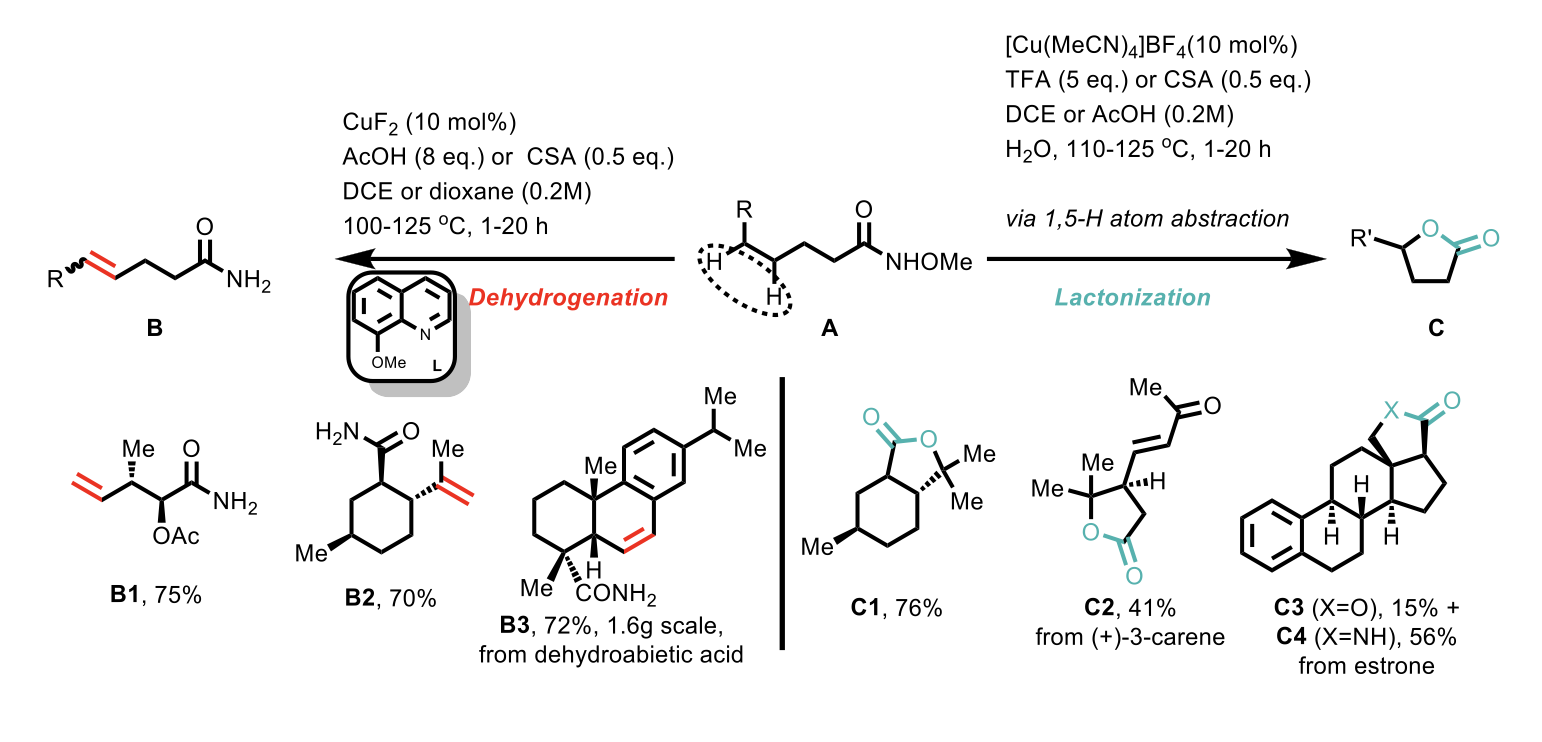
Scheme 4: Reaction conditions for the dehydrogenation (left-hand side) and lactonization (right-hand side) transformations.
The generality of this methodology is showcased by its effectiveness across a diverse range of substrates generating secondary and tertiary olefines via dehydrogenation in good yields and with good regio- and stereo- control.
For lactonization, the authors demonstrated generality of this transformation by using substrates with activated benzylic, allylic and propargylic moieties, as well as unactivated γ-C-H bonds. In addition, γ-lactones were preferentially formed even when more reactive benzylic C-H bonds were present.
For both pathways, the chemistry is very robust, scalable and works well with good yields even for complex natural product substrates (e.g. B3, C3, and C4 as shown in Scheme 4).
In conclusion, the authors have shown that this methodology gives direct access to valuable unsaturated amides and lactones with low catalyst loading; and excellent selectivity between dehydrogenation and lactonization when the right conditions are applied. Other merits of this approach included the usage of readily accessible N-methoxyamides and the use of no external oxidising agent, which makes it a more environmentally friendly strategy.
We hope you found this blog interesting. Our next review will be available soon, but in the meantime, get in touch to find out how we can help solve your synthetic chemistry challenges.