By the Domainex Synthesis Group (Hugh Tawell, Andrew Jones, Andrea Bombana, Venu Komanduri, James Harnedy, Brahmam Medapi, Robyn Presland, and Vinny Duong)
In this edition of our blog series, we have focused on the following two recent publications, which describe interesting and useful synthetic transformations:
- Boryl Radical Mediated Halogen Atom Transfer for Versatile C-C Bond Formation by Transition Metal Catalysed Cross Coupling. Zhenhua Zhang, Michael J. Tilby, and Daniele Leonori, Nat. Synth., 2024.
- Stereospecific alkenylidene homologation of organoboronates by SNV reaction. Miao Chen, Christian D. Knox, Mithun C. Madhusudhanan, Thomas H. Tugwell, Coco Liu, Peng Liu, and Guangbin Dong, Nature, 2024, 328-334, 631.
Boryl Radical Mediated Halogen Atom Transfer for Versatile C-C Bond Formation by Transition Metal Catalysed Cross Coupling
Transition metal catalysed cross-coupling reactions are a fundamental approach to C-C bond formation. In recent years, carbon centred radicals have become increasingly prevalent cross coupling partners, particularly under nickel and copper catalysis. Alkyl halides are attractive alkyl radical precursors, which can undergo halogen atom transfer (XAT), facilitated by an XAT reagent, resulting in the formation of alkyl radicals. Recent advances in this area have utilised silyl radicals and α-amino radicals as XAT reagents, however these reagents have limitations. Silyl radicals are often incapable of abstracting halogens from hindered halide substrates, limiting the scope to cyclic secondary alkyl halides. Whereas, α-amino radicals often compete with the desired alkyl radical in subsequent cross coupling reactions, leading to selectivity issues.
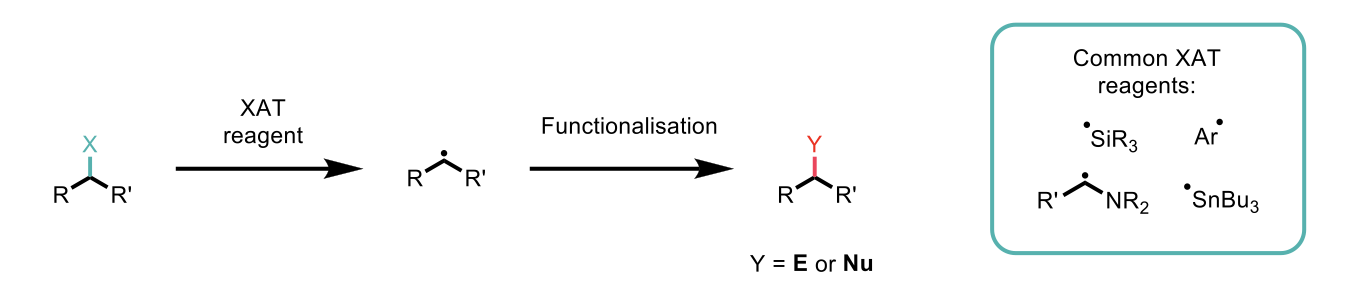
Scheme 1: Overview of radical formation using XAT reagents and subsequent reaction with an electrophile or nucleophile
Here, the authors report the use of amine-ligated boryl radicals as capable and versatile XAT reagents, with high selectivity for XAT over undesired hydrogen atom transfer (HAT) processes. The authors demonstrate the compatibility of this method of alkyl radical generation with both nickel catalysed cross-electrophile coupling, and copper catalysed coupling with nucleophilic boronic acids.
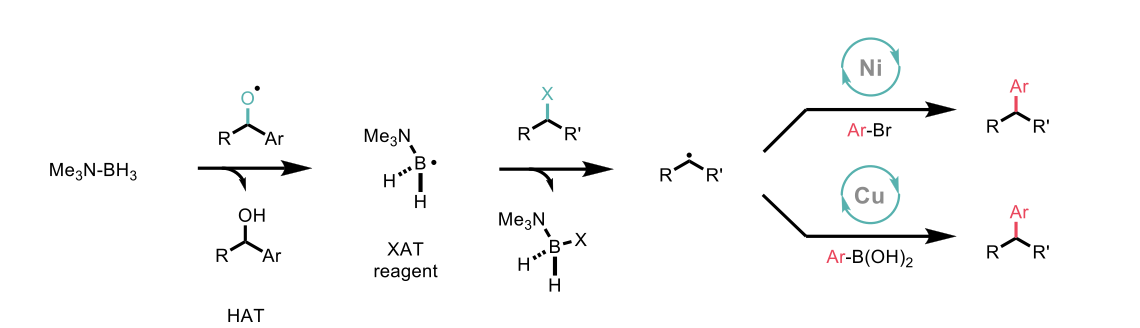
Scheme 2: Use of alkyl halides in divergent cross-coupling reaction aided by XAT activation
For the cross-electrophile coupling, a range of substituted aryl and heteroaryl bromides were shown to be effective in this transformation, and good functional group tolerance of esters, nitriles, and tetralones was demonstrated. The reaction was tolerant to a variety of alkyl iodide coupling partners, including primary and secondary acyclic alkyl iodides and numerous cyclic examples. Alkyl bromides were also tolerated, however the cross coupling proved harder to achieve and hence led to reduced yields of the desired products.
The copper-catalysed coupling of boronic acids with alkyl halides was shown to have an equally notable scope, with a range of aryl and heteroaryl boronic acids employed bearing electron donating and withdrawing groups, in conjunction with a range of primary, secondary and tertiary alkyl halides to afford a range of diverse cross coupled products.
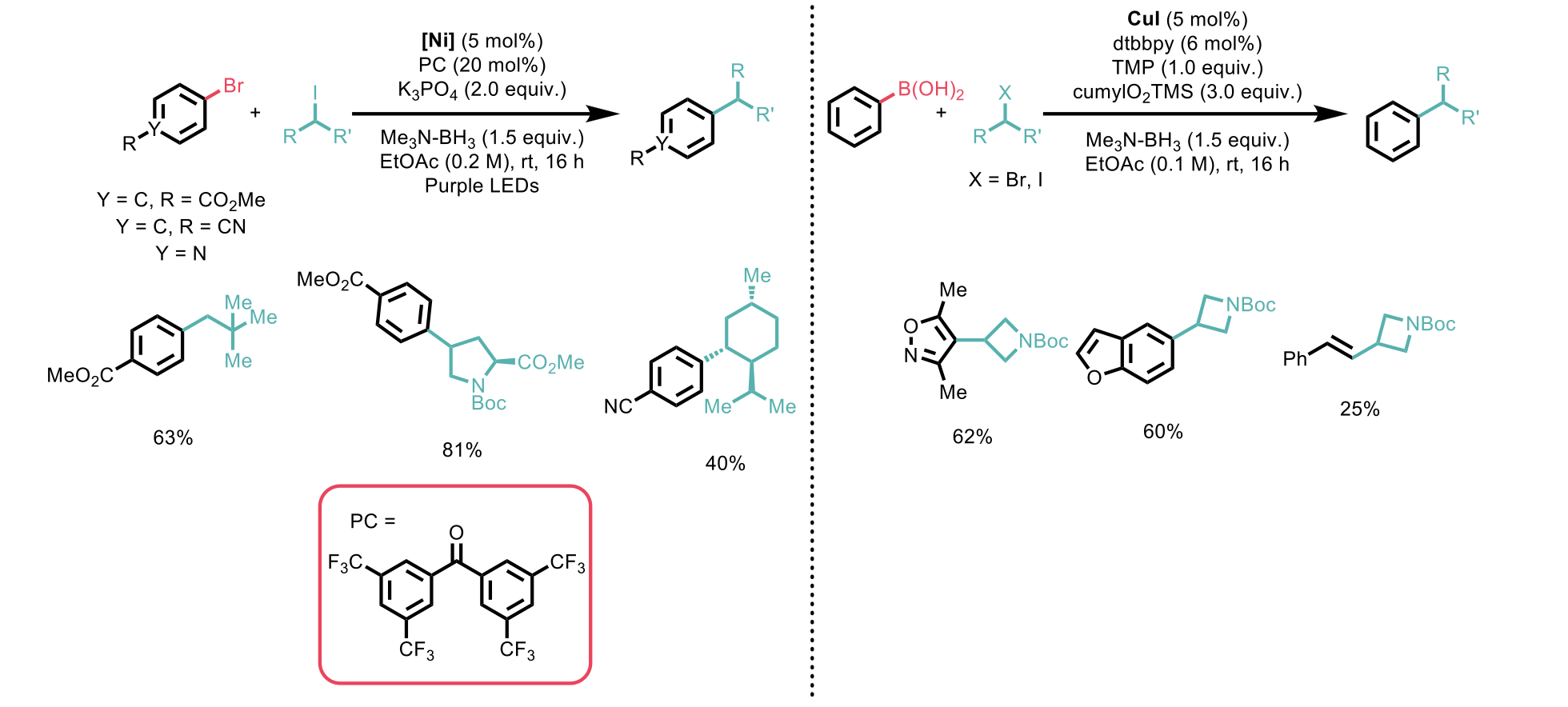
Figure 1: Examples of alkyl halide substrate scope for the cross-coupling reaction with electrophilic aryl bromides (left) and nucleophilic aryl boronic acids (right)
In conclusion, the authors have demonstrated that amine ligated boryl radicals are versatile HAT reagents and their reactivity is compatible with nickel and copper catalysis. This methodology has the potential to expand the scope of XAT transformations, allowing for the use of hindered alkyl halides in such transformations.
Stereospecific Alkenylidene Homologation of Organoboronates by SNV Reaction
Stereoinvertive nucleophilic substitution on electronically unbiased sp2 vinyl electrophiles, SNV(σ) reaction, is a concerted reaction analogous to the well understood and more commonplace concerted nucleophilic substitution reaction (SN2). Existing work on the SNV(σ) reaction has relied on purposely designed substrates utilized for ring formation. In their recent publication, Chen and co-workers reported a generic and stereospecific alkenylidene homologation method to access a wide range of organoboronates. The method utilizes concerted SNV reactions, accelerated by a proposed strain-release mechanism, in metalated complexes. The application of this methodology was expanded to the iterative incorporation of multiple alkenylidene units, achieving the challenging synthesis of cross-conjugated polyenes. Furthermore, the group demonstrated their method could be used in the synthesis of biologically significant compounds containing multi-substituted alkenes.
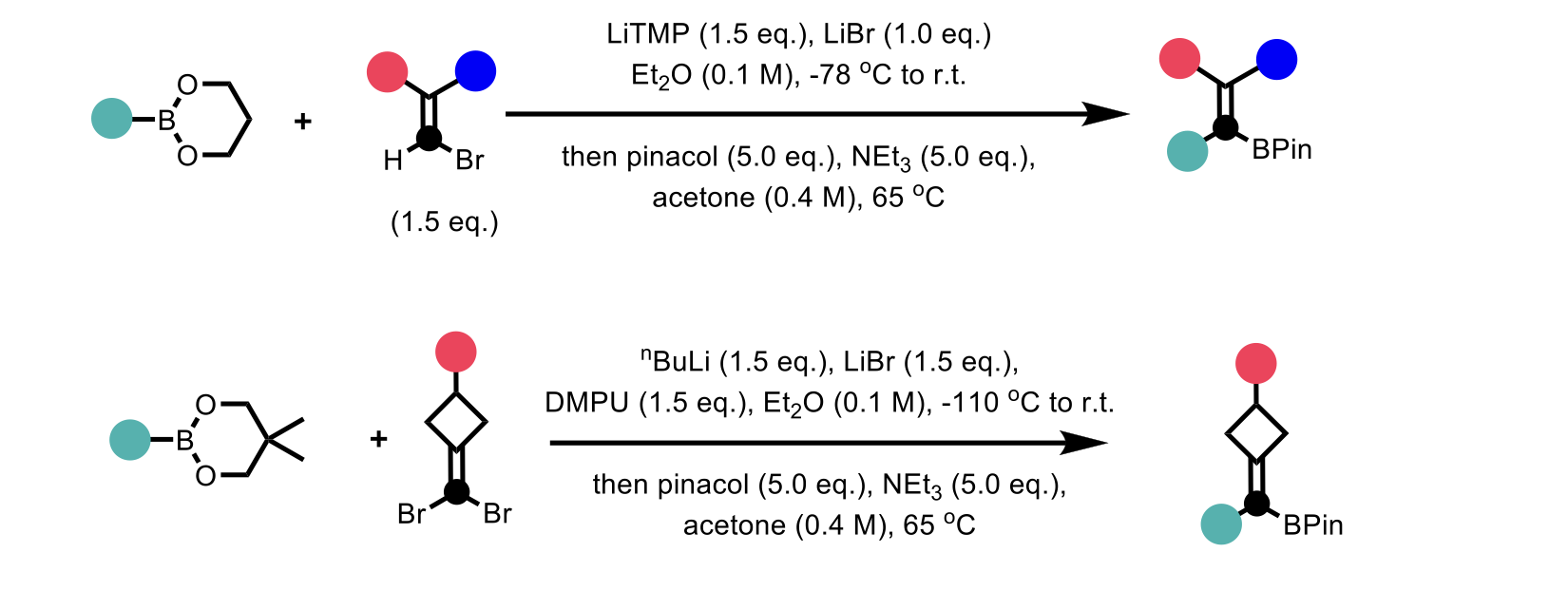
Scheme 3: Overview of stereospecific alkenylidene homologation
Efforts to optimize the alkenylidene homologation reaction found that the protecting group attached to the boron species had a significant impact on the reactivity, in general 6-membered boronates reacted preferentially compared to 5-membered boronates i.e. pinacol boronic esters. Reaction solvent was found to be critical with tetrahydrofuran (THF) affording markedly lower yields compared to diethyl ether. The reaction provided moderate to excellent yields with several substrates affording yields > 90%. In the case of symmetrical alkenylidene homologation, n-butyllithium was used for the lithium-halogen exchange deviating from lithium tetramethylpiperidide (LiTMP) used in the case of unsymmetrical alkenylidene homologation.
The group showed that both E and Z isomers of a diverse range of 2,2-disubstituted vinyl bromides undergo the desired transformation to the corresponding alkenyl boronates. Notably the reaction led to formation of the product as a single geometric isomer. A range of alkyl and aryl boronates, with different electronic properties, were tolerated and underwent the desired transformation in good to excellent yields. Although the group did observe that using the analogous alkenyl iodide as the coupling partner reduced the efficiency of the reaction.
The authors showed the applicability of this methodology to access bioactive compounds possessing multi-substituted alkenes. Z-Droloxifene and Tamoxifen are just two examples of bioactive compounds synthesised, both achieved in two steps with moderate to good yields.
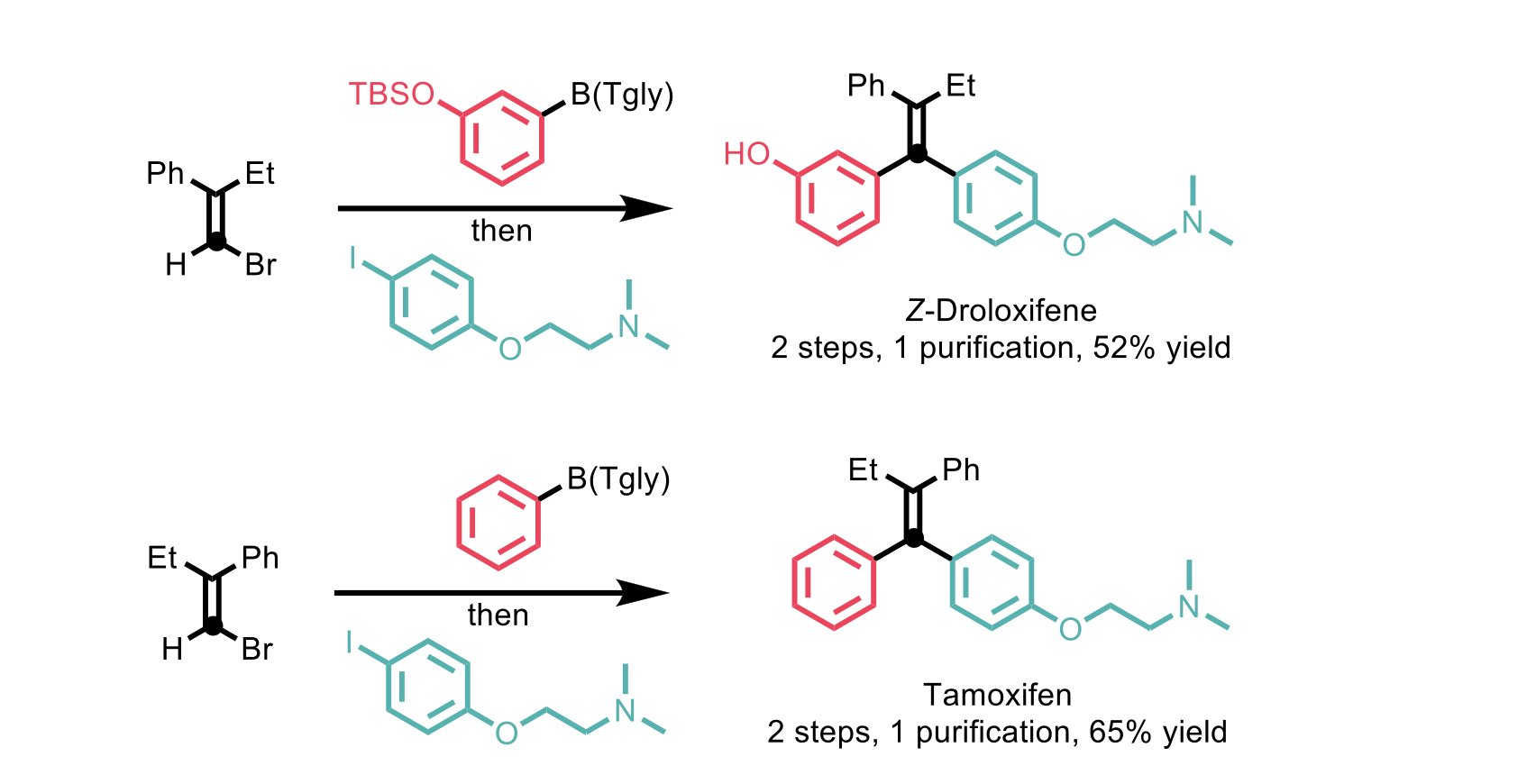
Scheme 4: Examples of bioactive compounds obtained via stereospecific alkenylidene homologation.
In conclusion, the authors successfully demonstrated an efficient, stereospecific and widely applicable alkenylidene homologation method enabled by a strain-release-enhanced metallate SNV mechanism. In the future, this work, notably the mechanistic understanding gained from computational studies of this reaction, could be utilized to develop further synthetically applicable homologation methods.
If you have an interesting synthetic or medicinal chemistry challenge you would like to discuss with Domainex’s experts, please get in touch.